HSDD has been a recognized medical condition for over 4 decades7


For US healthcare professionals only.
For US healthcare professionals only.
ABOUT HSDD


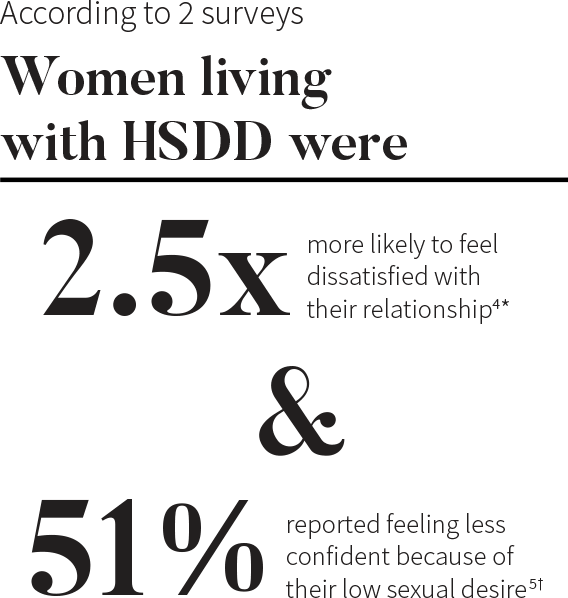
*Based on the WISHeS survey conducted February to May of 2000. The results shown above are from a US cohort of 129 women with HSDD.4
†Results of an attitudinal web-based survey in the US conducted in August 2012. Among the 1,914 women who responded to the survey, 450 reported low sexual desire and related distress.5
Changes in sexual desire are not always accompanied by observable changes in sexual behavior or frequency. Women with low sexual desire still engage in sexual activity, but it’s infrequent or initiated by a partner.3,4,6


INDICATION
VYLEESI is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder (HSDD), as characterized by low sexual desire that causes marked distress or interpersonal difficulty and is NOT due to:
Acquired HSDD refers to HSDD that develops in a patient who previously had no problems with sexual desire. Generalized HSDD refers to HSDD that occurs regardless of the type of stimulation, situation or partner.
Limitations of Use
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
VYLEESI is contraindicated in patients who have uncontrolled hypertension or known cardiovascular disease.
WARNINGS AND PRECAUTIONS
Transient Increase in Blood Pressure and Decrease in Heart Rate: VYLEESI transiently increases blood pressure and reduces heart rate after each dose. Advise patients that these changes usually resolve within 12 hours. VYLEESI is not recommended in patients at high risk for cardiovascular disease. Consider the patient’s cardiovascular risk before initiating VYLEESI and periodically during treatment and ensure blood pressure is well-controlled. To minimize the risk of more pronounced blood pressure effects, patients should not take more than one VYLEESI dose within 24 hours. Patients should not use more than 8 VYLEESI doses per month.
Focal Hyperpigmentation: Reported by 1% of patients who received up to 8 doses per month, including involvement of the face, gingiva and breasts. Patients are at higher risk of developing focal hyperpigmentation if they have darker skin and with daily dosing. Resolution of the focal hyperpigmentation was not confirmed in all patients after discontinuation of VYLEESI. Consider discontinuing VYLEESI if hyperpigmentation develops.
Nausea: Reported by 40% of patients who received up to 8 monthly doses, requiring anti-emetic therapy in 13% of patients and leading to premature discontinuation for 8% of patients. Nausea improves for most patients with the second dose. Consider discontinuing VYLEESI or initiating anti-emetic therapy for persistent or severe nausea.
ADVERSE REACTIONS
Most common adverse reactions (incidence >4%) are nausea, flushing, injection site reactions, headache, and vomiting.
DRUG INTERACTIONS
VYLEESI may slow gastric emptying and impact absorption of concomitantly administered oral medications. VYLEESI may significantly decrease the systemic exposure of orally-administered naltrexone; avoid use with orally administered naltrexone-containing products intended to treat alcohol or opioid addiction.
PREGNANCY
Advise patients to discontinue VYLEESI if pregnancy is suspected. Advise patients to use effective contraception while taking VYLEESI.
A pregnancy exposure registry monitors pregnancy outcomes in women exposed to VYLEESI during pregnancy. Pregnant women exposed to VYLEESI and healthcare providers are encouraged to call the VYLEESI Pregnancy Exposure Registry at 1-800-922-1038.
Please see full Prescribing Information.
References: 1. Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970-978. 2. Goldstein I, Kim NN, Clayton AH, et al. Hypoactive sexual desire disorder: International Society for the Study of Women’s Sexual Health (ISSWSH) expert consensus panel review. Mayo Clin Proc. 2017;92(1):114-128. 3. Parish SJ, Goldstein AT, Goldstein SW, et al. Toward a more evidence-based nosology and nomenclature for female sexual dysfunctions—part II. J Sex Med. 2016;13(12):1888-1906. 4. Leiblum SR, Koochaki PE, Rodenberg CA, Barton IP, Rosen RC. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women’s International Study of Health and Sexuality (WISHeS). Menopause. 2006;13(1):46-56. 5. Kingsberg SA. Attitudinal survey of women living with low sexual desire. J Womens Health. 2014;23(10):817-823. 6. Bancroft J, Loftus J, Long JS. Distress about sex: a national survey of women in heterosexual relationships. Arch Sex Behav. 2003;32(3):193-208. 7. Brotto LA. The DSM diagnostic criteria for hypoactive sexual desire disorder in women. Arch Sex Behav. 2010;39(2):221-239. 8. Angel K. The history of ‘female sexual dysfunction’ as a mental disorder in the 20th century. Curr Opin Psychiatry. 2010;23(6):536-541. 9. Kaplan HS. Hypoactive sexual desire. J SexMed Mar Ther. 1977;3:3-9. 10. McCabe MP, Sharlip ID, Atalla E, et al. Definitions of sexual dysfunctions in women and men: a consensus statement from the Fourth International Consultation on Sexual Medicine 2015. J Sex Med. 2016;13(2):135-143 11. US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research. Low sexual interest, desire, and/or arousal in women: developing drugs for treatment. Fed Reg. 2016;1(1):1-12. 12. Kingsberg SA, Clayton AH, Pfaus JG. The female sexual response: current models, neurobiological underpinnings and agents currently approved or under investigation for the treatment of hypoactive sexual desire disorder. CNS Drugs. 2015;29(11):915-933. 13. Holstege G. How the emotional motor system controls the pelvic organs. Sex Med Rev. 2016;4(4):303-328.
INDICATION
VYLEESI is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder (HSDD), as characterized by low sexual desire that causes marked distress or interpersonal difficulty and is NOT due to:
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
VYLEESI is contraindicated in patients who have uncontrolled hypertension or known cardiovascular disease.

INDICATION
VYLEESI is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder (HSDD), as characterized by low sexual desire that causes marked distress or interpersonal difficulty and is NOT due to:
Acquired HSDD refers to HSDD that develops in a patient who previously had no problems with sexual desire. Generalized HSDD refers to HSDD that occurs regardless of the type of stimulation, situation or partner.
Limitations of Use
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
VYLEESI is contraindicated in patients who have uncontrolled hypertension or known cardiovascular disease.
WARNINGS AND PRECAUTIONS
Transient Increase in Blood Pressure and Decrease in Heart Rate: VYLEESI transiently increases blood pressure and reduces heart rate after each dose. Advise patients that these changes usually resolve within 12 hours. VYLEESI is not recommended in patients at high risk for cardiovascular disease. Consider the patient’s cardiovascular risk before initiating VYLEESI and periodically during treatment and ensure blood pressure is well-controlled. To minimize the risk of more pronounced blood pressure effects, patients should not take more than one VYLEESI dose within 24 hours. Patients should not use more than 8 VYLEESI doses per month.
Focal Hyperpigmentation: Reported by 1% of patients who received up to 8 doses per month, including involvement of the face, gingiva and breasts. Patients are at higher risk of developing focal hyperpigmentation if they have darker skin and with daily dosing. Resolution of the focal hyperpigmentation was not confirmed in all patients after discontinuation of VYLEESI. Consider discontinuing VYLEESI if hyperpigmentation develops.
Nausea: Reported by 40% of patients who received up to 8 monthly doses, requiring anti-emetic therapy in 13% of patients and leading to premature discontinuation for 8% of patients. Nausea improves for most patients with the second dose. Consider discontinuing VYLEESI or initiating anti-emetic therapy for persistent or severe nausea.
ADVERSE REACTIONS
Most common adverse reactions (incidence >4%) are nausea, flushing, injection site reactions, headache, and vomiting.
DRUG INTERACTIONS
VYLEESI may slow gastric emptying and impact absorption of concomitantly administered oral medications. VYLEESI may significantly decrease the systemic exposure of orally-administered naltrexone; avoid use with orally administered naltrexone-containing products intended to treat alcohol or opioid addiction.
PREGNANCY
Advise patients to discontinue VYLEESI if pregnancy is suspected. Advise patients to use effective contraception while taking VYLEESI.
A pregnancy exposure registry monitors pregnancy outcomes in women exposed to VYLEESI during pregnancy. Pregnant women exposed to VYLEESI and healthcare providers are encouraged to call the VYLEESI Pregnancy Exposure Registry at 1-800-922-1038.
Please see full Prescribing Information.
to learn more about Vyleesi