CO-PRIMARY ENDPOINT #1
Significant increase in sexual desire
Women taking Vyleesi experienced a significant increase in desire compared to those taking placebo1
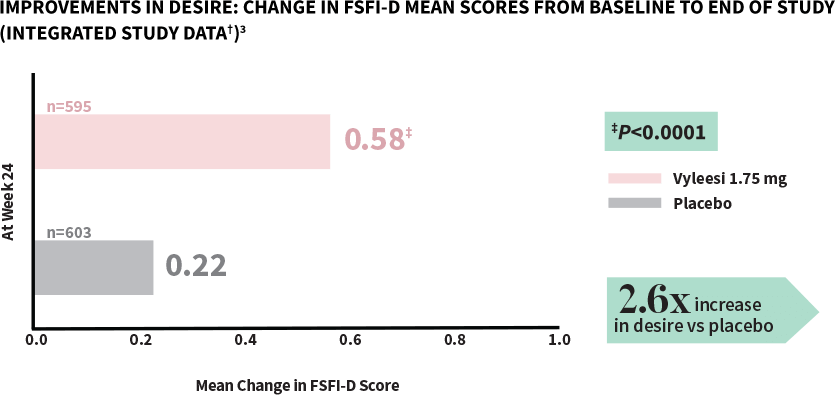
FSFI-D=Female Sexual Function Index-Desire Domain; FSFI=Female Sexual Function Index.
†Analysis based on MITT (modified intent to treat) defined as all subjects who were randomized, used at least 1 dose of the double-blind study drug, and had at least 1 double-blind follow-up visit.1
‡P-value from unadjusted Van Elteren test stratified by study.3
Women taking Vyleesi saw improvement in their low sexual desire at the first assessment—improvement continued over time1,3
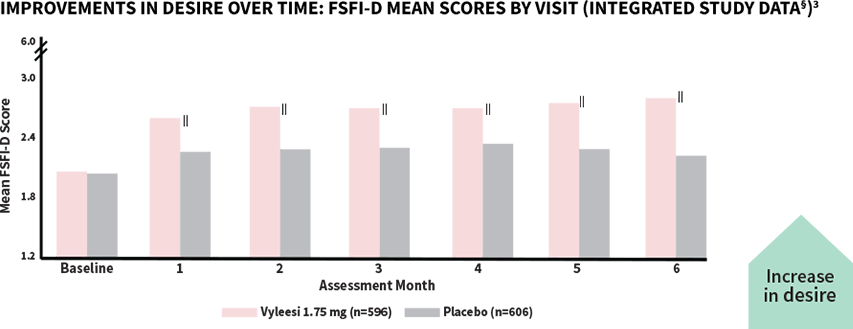
‖All Assessment Months were statistically significant—Months 1-6 (P<0.05).¶
The score for Vyleesi was 2.06 compared to placebo (2.04) at baseline. Scores range from 1.2 (lowest desire) to 6 (highest desire). The desire domain encompasses the first 2 questions in the FSFI.3
§Analysis based on MITT (modified intent to treat) defined as all subjects who were randomized, used at least 1 dose of the double-blind study drug, and had at least 1 double-blind follow-up visit.1
¶P-value from unadjusted Van Elteren test stratified by study.3
In October 2016, the FDA updated draft guidance for all clinical trials for hypoactive sexual desire disorder. Validated patient-reported outcomes (PROs) are appropriate to be used when evaluating change in sexual desire (FSFI-D) with interventions5
The FSFI is a 19-item, validated questionnaire used to measure overall sexual function in women. Sexual desire is measured using questions 1 and 2 within the Desire Domain (FSFI-D)5
In clinical trials, women are asked to rate desire using the Likert scale
Q1
Over the past 4 weeks, how often did you feel sexual desire or interest?
(Answer 1=almost never or never to 5=almost always or always)
Q2
Over the past 4 weeks, how would you rate your level (degree) of sexual desire or interest?
(Answer 1=very low or none at all to 5=very high)
The 2 response scores are summed, and raw scores are multiplied by a factor of 0.6, providing a sexual desire domain score that ranges from 1.2 to 6.0.
An increase in the FSFI-D domain score denotes improvement in sexual desire5


FDA=Food and Drug Administration.

CO-PRIMARY ENDPOINT #2
Significant decrease in distress
Women taking Vyleesi experienced a significant decrease in distress compared to those taking placebo1
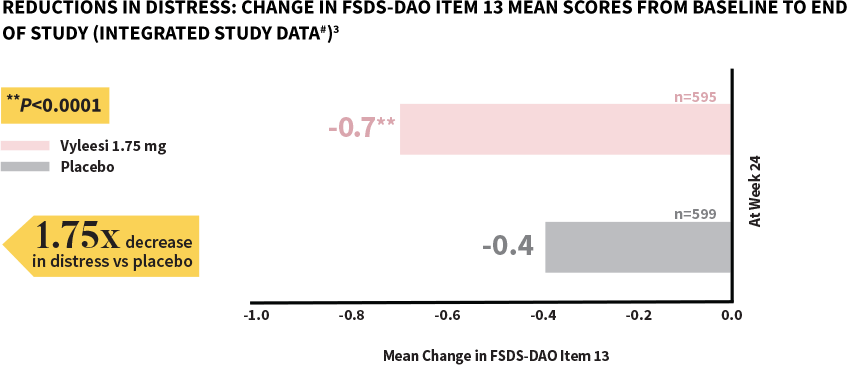
FSDS-DAO=Female Sexual Distress Scale-Desire/Arousal/Orgasm.
#Analysis based on MITT (modified intent to treat) defined as all subjects who were randomized, used at least 1 dose of the double-blind study drug, and had at least 1 double-blind follow-up visit.1
**P-value from unadjusted Van Elteren test stratified by study.4
Women taking Vyleesi saw a reduction in distress at the first assessment—decrease continued over time1,3
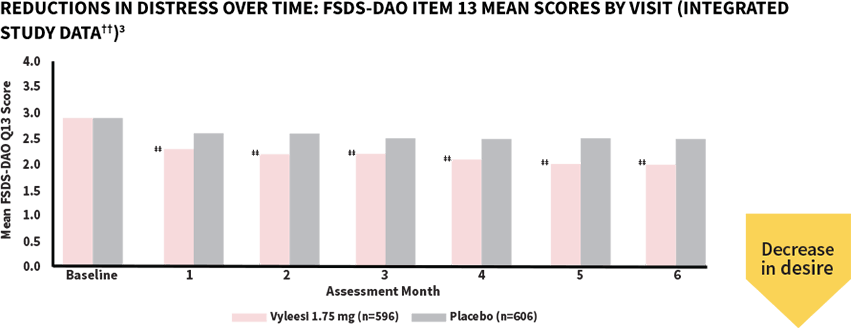
‡‡All Assessment Months were statistically significant—Months 1-6 (P<0.05).§§
The score for both treatment groups was 2.9 at baseline. Scores ranged from 0 (Never feel bothered) to 4 (Always feel bothered). Distress related to sexual desire is measured using Question 13 of the FSDS-DAO.3,5
††Analysis based on MITT (modified intent to treat) defined as all subjects who were randomized, used at least 1 dose of the double-blind study drug, and had at least 1 double-blind follow-up visit.1
§§P-value from unadjusted Van Elteren test stratified by study.3
In October 2016, the FDA updated draft guidance for all clinical trials for hypoactive sexual desire disorder. Validated patient-reported outcomes (PROs) are appropriate to be used when evaluating change in personal distress (FSDS-DAO Item 13) with interventions5
The FSDS-DAO is a 15-item, validated questionnaire used to measure different aspects of sexual distress. Question 13 asks women to rate their level of distress related to low sexual desire5,6
In clinical trials, women are asked to rate distress using the Likert scale
Q13
How often did you feel bothered by low sexual desire?
(Answer 0=never to 4=always)
A decrease in the FSDS-DAO Q13 score denotes improvement in the level of distress associated with low sexual desire5


FDA=Food and Drug Administration.

KEY SECONDARY ENDPOINT
Number of satisfying sexual events (SSEs)
There were no statistically significant differences between treatment groups and placebo in the change in the number of SSEs3
The Food and Drug Administration (FDA) no longer requires the number of SSEs to be a co-primary endpoint in clinical trials because it is not part of the diagnosis of HSDD.5
HSDD=hypoactive sexual desire disorder.
STUDY DESIGN
Over 1,200 women were involved in the pivotal trials
Clinical data included 2 identical, 24-week, randomized, double-blind, placebo-controlled core studies and 2 voluntary, 52-week, open-label extension studies.1,3,7
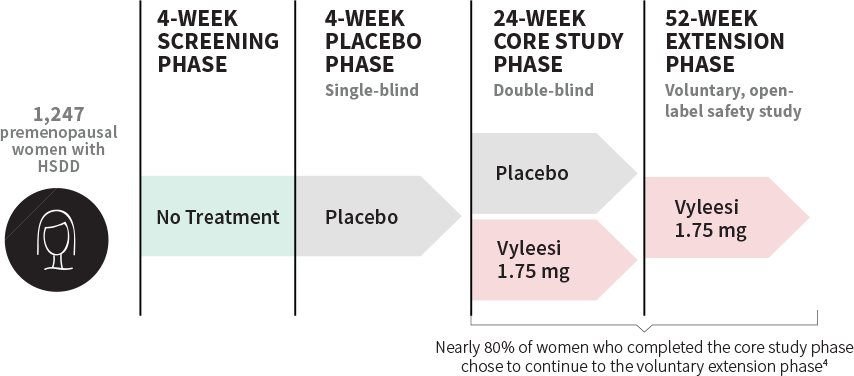
Baseline characteristics1
- 19-56 years old (premenopausal); average age was 39
- Mean duration of 4 years with acquired, generalized HSDD
- Racial demographics: Caucasian (86%), Black (12%)
- Female, ≥18 years of age, and premenopausal
- Had previously experienced a normal level of desire for a period of at least 2 years
- Willing to engage in sexual activity at least once a month during the study
- In a stable relationship for at least 6 months
- Satisfied the diagnostic criteria for HSDD (per Diagnostic Screening Guide for HSDD) for at least 6 months
- Not pregnant
- On birth control for 3 months before first screening, continuing for the duration of the study and 1 month following end of study (heterosexual couples)
- Postmenopausal
- Pregnant or nursing
- Had a history of psychiatric or mental illness and/or alcohol/substance abuse (within 6 months before screening)
- Used medications: testosterone (within 6 months before screening), neuroleptics, lithium, antidepressants, mood stabilizers, benzodiazepines, stimulants, and certain nutritional supplements (within 3 months before screening).
HSDD=hypoactive sexual desire disorder.


