VYLEESI is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder (HSDD), as characterized by low sexual desire that causes marked distress or interpersonal difficulty and is NOT due to:
- A co-existing medical or psychiatric condition,
- Problems with the relationship, or
- The effects of a medication or drug substance.
Acquired HSDD refers to HSDD that develops in a patient who previously had no problems with sexual desire. Generalized HSDD refers to HSDD that occurs regardless of the type of stimulation, situation or partner.
Limitations of Use
- VYLEESI is not indicated for the treatment of HSDD in postmenopausal women or in men.
- VYLEESI is not indicated to enhance sexual performance.
CONTRAINDICATIONS
VYLEESI is contraindicated in patients who have uncontrolled hypertension or known cardiovascular disease.
WARNINGS AND PRECAUTIONS
Transient Increase in Blood Pressure and Decrease in Heart Rate: VYLEESI transiently increases blood pressure and reduces heart rate after each dose. Advise patients that these changes usually resolve within 12 hours. VYLEESI is not recommended in patients at high risk for cardiovascular disease. Consider the patient’s cardiovascular risk before initiating VYLEESI and periodically during treatment and ensure blood pressure is well-controlled. To minimize the risk of more pronounced blood pressure effects, patients should not take more than one VYLEESI dose within 24 hours. Patients should not use more than 8 VYLEESI doses per month.
Focal Hyperpigmentation: Reported by 1% of patients who received up to 8 doses per month, including involvement of the face, gingiva and breasts. Patients are at higher risk of developing focal hyperpigmentation if they have darker skin and with daily dosing. Resolution of the focal hyperpigmentation was not confirmed in all patients after discontinuation of VYLEESI. Consider discontinuing VYLEESI if hyperpigmentation develops.
Nausea: Reported by 40% of patients who received up to 8 monthly doses, requiring anti-emetic therapy in 13% of patients and leading to premature discontinuation for 8% of patients. Nausea improves for most patients with the second dose. Consider discontinuing VYLEESI or initiating anti-emetic therapy for persistent or severe nausea.
ADVERSE REACTIONS
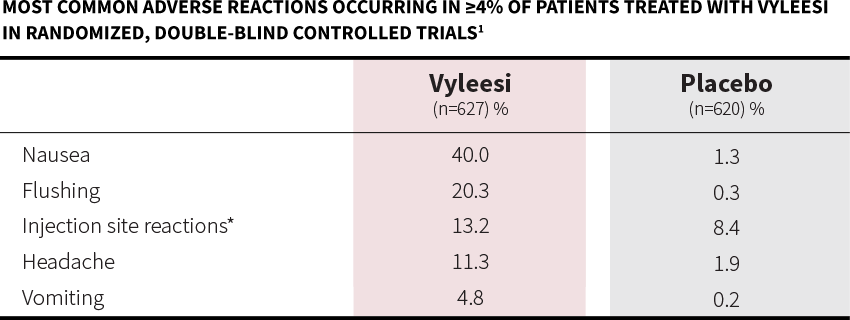
Most common adverse reactions (incidence >4%) are nausea, flushing, injection site reactions, headache, and vomiting.
DRUG INTERACTIONS
VYLEESI may slow gastric emptying and impact absorption of concomitantly administered oral medications. VYLEESI may significantly decrease the systemic exposure of orally-administered naltrexone; avoid use with orally administered naltrexone-containing products intended to treat alcohol or opioid addiction.
PREGNANCY
Advise patients to discontinue VYLEESI if pregnancy is suspected. Advise patients to use effective contraception while taking VYLEESI.
A pregnancy exposure registry monitors pregnancy outcomes in women exposed to VYLEESI during pregnancy. Pregnant women exposed to VYLEESI and healthcare providers are encouraged to call the VYLEESI Pregnancy Exposure Registry at 1-800-922-1038.
Please see full Prescribing Information.